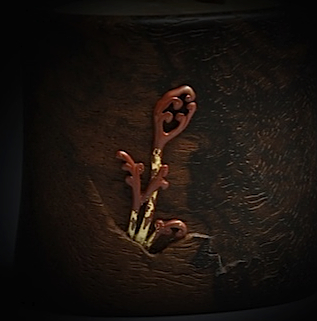
| Red Copper Patina using rokusho (hiirodo or hido) | ||||
| home Japanese Alloy Basics copyright 2010 Jim Kelso | ||||
| Other Tutorials Recent Works To Archives & Jewelry | ||||
|
||||
This is an addendum to my Japanese rokusho patina turorial found HERE Twenty five years ago or so I started noticing pieces of Japanese metalwork that were either primarily copper or had a copper element along with other alloys such as shibuichi, shakudo, etc. In these pieces the copper was a very rich red, much deeper red that you usually get with copper in the usual rokusho irotsuke (Japanese traditional soft-metal alloy patination). My earliest enquiry led to a recommendation to heat the piece to get a copper oxide which then was quenched in water leaving a deep red. I tried this half-heartedly, getting mixed results, as I suspected this wasn't really what I was after, as the pieces I had seen were a mix of the usual alloys found in sword fittings, along with the copper, and that was what I really wanted. Heating a piece to get copper oxide would play hell with the rest of the alloys, and I knew there must be some other way. When I met my teacher Toshimasa, this was one of the things I asked about as he had used hiirodo (as he calls it) on one tsuba ( (see below). He said he used "pure copper" and gave me a piece that had some connection to the Shinkansen or Bullet Train. I put aside the quest for various reasons until a year or two ago, after seeing a kogo by Shoami Katsuyoshi and asking my friend Murata san, the owner, if he knew how it was patinated. He replied that our friend Katayama Shigeki said it is "pure copper" boiled in the usual solution but for ten hours. "Pure copper" is a term that in context can mean many things, as pure is almost always a relative term. I decided that I would try it with a piece of 110 copper alloy(99.9% pure, I think) and another piece of undetermined commercial sheet copper. Both of these pieces came out with very much the same color, after ten hours. I'm attaching the photo of the undetermined, but presumably relatively pure copper. It was photographed in natural daylight and I did nothing to the photo except size it. The red on the thumbtack box is pinkish as it appears. Around the eight hour mark the color started shifting from orangeish to redder. I want to boil one piece for some more time to see what happens. I've compared this color to the Katsuyoshi kogo and on my monitor, they are very close. I used this test result to proceed with confidence in the patination of the fern crosier seen above. Some further patina process details include: The color seems to develop consistently first to a pumpkin or terra cotta shade within an hour or so, then to a nutty brown going to a dark orange from 2-4 hours and then from 6-10 hours gradually turning red. A logical concern is how the other alloys would fair during such a long bath, if the piece had one or more alloys beside the copper. When I made the test described above, I put in a piece of shakudo as a test also. It was at it's full color within an hour so I was anxious to see how it would stand up over ten hours. I had a little problem with a residue buildup on the shakudo as the pieces were laying somewhat flat in the bath for so long, but the color was fine. Normally I tend the process like a nervous hen, which includes swirling the rokusho off the pieces(it doesn't fully disolve), but I couldn't do this over ten hours. Oddly, this residue buildup was not a problem on the copper. As I said, the color was fine on the shakudo after ten hours, and the residue problem can be solved by suspending the piece so it presents as little horizontal surface as possible and occasionally brushing the rokusho off with a small brush(while in the solution). I didn't try a shibuichi sample but I'm sure it will be fine because there are historical examples, as on this page, with all the alloys present that look great. So the answer is yes, it will redden the copper, and leave the other alloys as usual. I would say also that if there was some minor problem with the other alloys such as slight yellowing on silver or shibuichi, it should not in most cases be terribly difficult, once the copper is red, to back up and lightly, selectively polish the other alloys, return the piece to the bath and get everything looking just about perfect.
|
||||
|
||||